D-dimer levels as a procoagulative marker in association with disease progress during giardiasis in dogs
D dímero como marcador procoagulante en asociación con el progreso de la enfermedad durante la giardiasis en perros
D-dimer levels as a procoagulative marker in association with disease progress during giardiasis in dogs
Revista MVZ Córdoba, vol. 23, no. 2, 2018
Universidad de Córdoba
Received: 07 August 2017
Accepted: 04 December 2017
Abstract: Objective. The present study was conducted to measure D-dimer concentrations and assess their value in disease activity in dogs with giardiasis. Furthermore another purpose was to analyze correlation between cyst excretion and D-dimer levels to those of dogs naturally infected with Giardia sp. Materials and methods. D-dimer analysis were performed in three groups of dogs; (i) 15 dogs with giardiasis to those of treated with secnidazol, (ii) 10 dogs with giardiasis, left untreated as control group, then were compared to those of (iii) 17dogs without giardiasis, used to detect reference ranges for D-dimer values as control group. was a correlation between D-dimer levels and logarythmic cyst counts. Results. The D-dimer range in healthy dogs was <0.1 mg/L. In dogs with giardiasis, the D-dimer concentrations were greater than those of healthy dogs (p<0.05) and (p<0.01), respectively. The mean initial plasma D-dimer level was 2.84±0.50 and 2.99±0.61 ng/L in treated and untreated control groups. At the final follow-up evaluation on day 10 was 0.27±0.50 and 2.14±0.61 ng/L, in treated and untreated control groups, respectively, which was significantly lower in treated group (p<0.001). The area under curve (AUC) of the receiver operating characteristics for d-dimer was 0.922 (z-value = 12.977, p<0.0001). (95%CI: 0.780–0.885). At a cut-off value of 0.1 ng/L, the D-dimer measurement had a sensitivity of 87.2%, a specificity of 90.9%. Conclusions. As a result D-dimer concentrations measured in giardiasis support the probable link between probable pro-thrombotic and inflammatory condition.
Keywords: D-dimer, procoagulation, inflammation, biomarker, giardiasis, dogs.
Resumen: Objetivo. El presente estudio se realizó para medir las concentraciones del dímero D y detectar su valor en la actividad de la enfermedad en perros con giardiasis. Además, otro objetivo fue analizar la correlación entre la excreción de quistes y los niveles de D-dímero a los de perros naturalmente infectados con Giardia sp. Materiales y métodos. El análisis del dímero D se realizó en tres grupos de perros; (I) 11 perros con giardiasis tratados con secnidazol, (ii) 10 perros con giardiasis, no tratados como grupo control, luego se compararon con los de (iii) 17 perros sin giardiasis, utilizados para detectar rangos de referencia para el dímero D Valores como grupo de control. Resultados. El rango del D-dímero en perros sanos fue <0.1 mg/L. En perros con giardiasis, las concentraciones de dímero D fueron mayores que las de perros sanos (p<0.05) y (p<0.01), respectivamente. El nivel medio inicial de dímero D plasmático fue 2.84±0.50 y 2.99±0.61 ng/L en los grupos de control tratados y no tratados. En la evaluación final de seguimiento al día 10 se obtuvieron 0.27±0.50 y 2.14±0.61 ng/L, tratados y no tratados, respectivamente, que fue significativamente menor en el grupo tratado (p<0.001). El área bajo curva (AUC) de las características de funcionamiento del receptor para el dímero d fue 0.922 (valor z = 12.977, p<0.0001). (IC del 95%: 0.780-0.885). Con un valor de corte de 0.1 ng/l, la medida del dímero-D tenía una sensibilidad de 87.2%, una especificidad de 90,9%. Hubo una correlación entre los niveles de D-dímero y los recuentos de quistes logarítmicos. Conclusiones. Como resultado, las concentraciones del dímero D medidos en la giardiasis apoyan el probable vínculo entre la probable condición pro-trombótica e inflamatoria.
Palabras clave: D-dímero, procoagulación, inflamación, biomarcador, giardiasis, perros.
INTRODUCTION
The understanding immunity and pathogenesis of giardiasis has been notably developing, due to disparity in several investigations persist, remaining lot to be learned (1,2). Different parasites have long been recognized to affect various aspects of their host’s pro-inflammatory responses (3,4,5). Furthermore available evidence (i.e. findings from selected human researches and to those of experimental proof) suggested that Giardia sp., are able to modulate host pro-inflammatory response (6, 7). The latter knowledge has to be taken into consideration as giardiasis is frequently detected in relation with a variety of pro-inflammatory gastrointestinal (GI) pathogens. Human patients with chronic assemblage B infection were detected to present duodenal inflammation (6). “In vivo” studies showed that experimental infections with G. duodenalis assemblage B were related to post-infectious neutrophil infiltration in ileum or intestinal inflammatory response (8,9). Investigation polarated from human (10,11), and ruminants (12), suggested that some of the Giardia infections might be related to eosinophilia; as was also described previously by “in vivo” infections with Giardia assemblage B H3 infections (13) or parasite excretory/secretory products (14). Duodenal inflammation (6) as detected by microscopy was detected in human patients post-giardiasis (15). Results from various studies indicated roles of specific pro-inflammatory cytokines in giardiasis (16). Proinflammatory conditions in critically ill hospitalized human caused increased D-dimer levels via cytokine activation of the coagulation cascade and subsequent inhibition of fibrinolysis (17). Contrarily no such relation has been made among D-dimer levels, proinflammatory cytokines and giardiasis.
The elevated incidence of thromboembolic events in Inflammatory Bowel Disease (IBD) seems like having multifactorial etiology, where as the inflammatory process might itself participate (18, 19). Furthermore, a defective or impaired intestinal mucosa barrier function may as well contribute to the procoagulative state, especifically in humans with IBD (20, 21). The pathogenic mechanisms proposed for Giardia sp. infections also composed of induction of IBD (22, 23). To this context it may not be unwise to speculate that probable IBD in canine giardiasis might be promoting to a procaogulative state. The alteration of intestinal barrier function in IBD (21), and giardiasis (22,23,24,25,26,27,28) may be a cofactor promoting a procoagulative state.
Relevant data strongly suggested the persence of multifactorial post-infectious complications in giardiasis. One area of developing interest in giardiasis is that initiation of delayed immune-mediated pathophysiology during the acute stage of the infection. Furthermore a better knowledge of the conditions responsible for post-infectious manifestations of giardiasis might help explore common pathways leading to this disease to this context, a biochemical parameter, such as D-dimer, would have helped better understanding the relationship between probable inflammation and thrombosis. This may be briefly explained by some selected hypoythesis. Given the relationship between D-dimer and C-reactive protein, indicating a connection between inflammation and thrombosis (29), each quartile in which C-reactive protein increased, there was a correlational elevation in D-dimer. The latter correlation suggested that as inflammation progresses, the likelihood of thromboembolism rises (29). Previous studies have investigated the responsible mechanisms in which inflammation might potentiate blood clotting. Inflammatory mediators might cause the expression of tissue factor on monocytes and, probably, endothelium, there by stimulating the coagulation cascade. Inflammation-induced thrombosis has long been recognized, indeed its pathogenesis remains complicated. Complex interactions between inflammation and hemostasis, composed of proinflammatory cytokines, and other relevant mediators might occur. Inflammation elevates procoagulant factors, additionally inhibits natural anticoagulant pathways and fibrinolytic activity, resulting with a thrombotic tendency.
To the present authors’ knowledge no such correlation regarding probable thrombosis and inflammation in dogs with giardiasis were detected previously. To verify whether dogs might display a prothrombotic condition, plasma D -dimer was assessed for its value in inflammatory disease activity in dogs with giardiasis. A furthermore another purpose was to analyze correlation between cyst excretion and D-dimer levels to those of dogs naturally infected with Giardia sp.
MATERIALS AND METHODS
Demographic data. All clinical records from November 2014 to January 2017 were searched to identify dogs with giardiasis for which Snap Giardia test kit analysis (Figure 1b), microscopical detection and a D-dimer test had been performed. A total of 51 cases were found that solely 25 met the criteria for involvement in the present study. These cases were then classified into groups I, II and III.
Classification of the dogs. Three groups of dogs were studied: group I consisted of 15 dogs naturally infected with giardiasis treated with antigiardial protocole (secnidazole at a dose of 10 mg/kg at a single oral dose). Group II consisted of 10 dogs naturally infected with giardiasis without any treatment application, left as untreated control. Finally group III (n=17) were composed of those of healthy dogs, without giardiasis, presented for routine health screen and physical examination, without any apparent clinical signs.
Study duration. The study was performed in 10 days duration. Day 0 (D0) was designated as the initial day, prior to a single dose treatment, and Day 10 (D10) was designed as after treatment analysis day.

Laboratory analysis
Fecal Examination. During the allocation period all dogs (Figure 1a showed one of them) were screened twice with a 10 days interval to confirm the presence/absence of Giardia sp. cysts (Figure 1f). For an initial diagnosis two native smear was prepared to those of dogs with altered fecal consistency (Figure 1c). This was followed by fecal material mixed with 33% ZnSO4 solution, added on to centrifuge tubes, which then was consequently spunned in centrifuge at 880 x g for 300 seconds, similary to a prior study (30). Following centrifugation, some of the fecal mixture was separated, then were added on a microscope slide containing Lugol iodine, which was covered by a slip. The slide was microscopically examined under 40x power for detecting Giardia cysts by a specialized parasitologist. To those of solely mono infected dogs with giardiasis were proven only by microscopical examination was included. Furthermore Giardia antigens in canine feces were tested by use of a rapid enzyme immunoassay for detection of (SNAP ® Giardia Test, IDEXX, USA), (Figure 1b).
Methodology for point of care D-dimer analysis. Two mililiters of sera samples were withdrawn from the vena cephalica of each dog into plain tubes without anticoagulant. Circulating D-dimer concentration was analyzed by use of the Point-of-Care fluorescent immunoassay (Figure 1d). The presentauthor’s private analytic room was specialized and utilized for the Wondfo Finecare FIA meter (FIAm) Fluorescence Immunoassay Rapid Quantitative Test, manufactured by; Guangzhou Wondfo Biotech Co. Ltd.; imported by RDA Grup, Turkey) which is automated. The use of FIAm D-dimer analyzer (Figure 1d, e) has been validated in veterinary practice in the present authors experience since 2014.
Briefly, the FIAm utilizes a Fluorescence Immunoassay methodology, in which 10 μl of undiluted serum is required. A human anti-D-dimer monoclonal antibody was implanted into the well on a supplied test cartridge via the manufacturer. Obtained sera sample were then added; along with buffer solution, which were then shaked for half a minute. Out of those mixtures, 75 μL was obtained, put on to the reading stribes and finally forwarded to the analyzer. The required duration for reading was 180 seconds, also composed of incubation. FIAm D-dimer analyzer is currently able to read ranges between 0.1-10 mg/L. Methodology was adapted with in the manufacturer directions. Elevated D-dimer levels was set as (>0.1mg/L).
Statistical Analysis. Descriptive statistics of the patient and control groups were obtained as follows Unpaired Two-way analysis of variance was performed to determine the differences between groups. ROC (reciever operator characteristics curve) analysis method was used for predicting cutt-off values among D-dimer levels. Results were deemed statistically significant at p<0.05 with 95% confidence interval. Regarding cyst counts on the basis of the number of 0 versus, 0 plus in dogs with giardiasis defining measurement of youden ROC analysis index >0.09 cuttoff value was determined. The average data for performing two-way analysis of variance between groups, and the number of cysts were subjected to log transformation of the time domain. Charts were presented as the median number of cysts.
RESULTS
D-dimer levels in dogs with giardiasis. Median D-dimer levels to those of dogs with giardiasis before (day 0) and after (day 10) treatment. Secnidazol treatment and untreated control groups were involved. Boxplots of median D-dimer levels in dogs with giardiasis before (day 0) and after (day 10) treatment were shown in Figure 2 (Table 1).
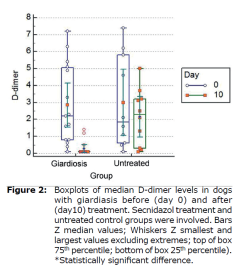
| Effect | Factor | Log Cyst (/g) Mean± SE (95% CI) | d-dimer (ng/L) Mean± SE (95% CI) | |
| Group x Time Interaction | Untreated | 0 | 5.35±0.27 (4.81-5.88) | 2.99±0.61 (1.76-4.22) |
| 10 | 5.18±0.26 (4.64-5.71) | 2.14±0.61 (0.91-3.37) | ||
| Treated | 0 | 5.28±0.21 (4.85-5.72) | 2.84±0.50 (1.84-3.85) | |
| 10 | 0.85±0.21 (0.42-1.30) | 0.27±0.50 (0.72-1.28) | ||
| p | <0.001 | 0.13 | ||
ROC curves analyses (Figure 3) were used to select D-dimer cutoff points of below 0.1 mg/L. The FIA meter assay had high specificity (90.9 %) at the 0.09 mg/L cutoff and relatively high sensitivity (87.2%) at the 0.09 mg/L cutoff, as shown in Figure 3 (Table 2).
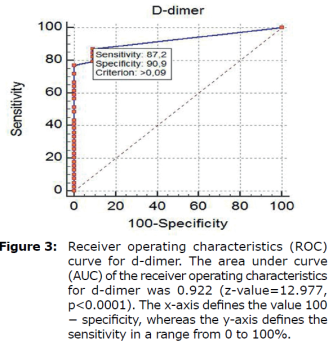
| Threshold Value1 (ng/l) | Sensitivity2 (95% CI) | Specificity3 (95% CI) | Likelihoodratio (95% CI) | Predictivevalue (95% CI) | P-value | ||
| Positive4 | Negative5 | Positive6 | Negative7 | ||||
| >0.09 | 87.18 (72.6-95.7) | 90.91 (58.7-99.8) | 9.59 (8.7-9.9) | 0.14 (0.1-0.2) | 97.16 (85.1-99.9) | 66.38 (38.2-87.9) | <0.01 |
| 1 Threshold value for d-dimer determined by the receiver operating characteristic (ROC) analysis. 2 Sensitivity: Probability that a test result will be positive when 0thedisease is present (truepositive rate). 3 Specificity: Probability that a test result will be negativewhenthedisease is not present (truenegative rate). 4 Positivelikelihoodratio: Ratio between the probability of a positive test result given the presence of the disease and the probability of a positive test result given the absence of the disease. 5 Negative likelihood ratio: Ratio between the probability of a negative test result given the presence of the diseaseand the probability of a negative test result given the absence of the disease. 6 Positive predictive value: Probability that the disease is present when the test is positive. 7 Negative predictive value: Probability that the disease is not present when the test is negative | |||||||
NOTE: * Area Under Curve (AUC), the following is interpreted:
90-1.00 = excellent
80-.90 = good
70-.80 = medium
60-.70 = weak
50-.60 = failed
Therefore, the AUC value of 0.922 obtained in this test can be considered excellent.
*The greater the positive portion of Likelihoodratio, the smaller the negative portion, the higher the reliability. Therefore, 9.59 + LR and 0.14-LR are quite good results.
*Treshold value means that animals with values greater than 0.09 ng/L are likely to be sick.
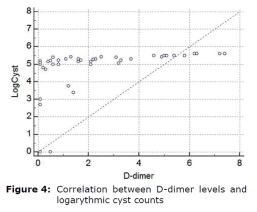
Correlation between D-dimer levels and logarythmic cyst counts were shown in figure 4.
DISCUSSION
Giardia infections cause acute or chronic diarrhea, dehydration, abdominal disturbance and weight loss. Despite suffering from Giardia’s disease, the underlying pathophysiological characteristics of the intestine and the disturbance in Giardia’s disease are not fully understood.
Giardiasis does not spread to other parts of the gastrointestinal tract, such as blood, but is limited to the lumen of the small intestine.
Interestingly, epithelial abnormalities responsible for intestinal malabsorption and responsible for diarrhea in giardiasis share similarities with those observed in other enteric disorders such as bacterial enteritis, small intestine bacterial over growth and chronic food allergies. It is understood that the pathology of the Giardia has emerged. These include disruption of the epithelial barrier, defects at the epithelial brush border, secretion of chloride ions, and hypermotility of the intestinal smooth muscles. The role of parasite virulence factors and host immune responses in these mechanisms has begun to be clarified, but much remains to be understood. The understanding immunity and pathogenesis of giardiasis has been notably developing, due to disparity in several investigations persist, remaining lot to be learned (1, 2). Different parasites have long been recognized to affect various aspects of their host’s pro-inflammatory responses (3,4,5). Furthermore available evidence (i.e. findings from selected human researches and to those of experimental proof) suggested that Giardia sp., are able to modulate host pro-inflammatory response (6, 7). The latter knowledge has to be taken into consideration as giardiasis is frequently detected in relation with a variety of pro-inflammatory gastrointestinal (GI) pathogens. Proinflammatory cytokines are generally released by activated macrophages and are important in the inflammatory response. It has been suggested that D-dimer activation, which is induced by the activation of clotting factors due to the activation of inflammatory processes in this disease, might briefly support our findings as a preliminary marker. In this study, we demonstrated by statistical analysis that d-dimer concentrations, which are considered as proinflammatory markers, are important and specific in this disease by stimulating inflammatory processes after arithmetic and geometric averages of cyst counts before and after treatment. The interactions between log cyst count and circulating D-dimer levels before (day 0) and after (day 10) treatment was interesting. Log cyst count per gram of feces (mean±SE, min-max at 95%CI) was compared to those of D-dimer levels at 95% confidence interval. The D-dimer range in healthy dogs was <0.1 mg/L. In dogs with giardiasis, the D-dimer concentrations were greater than those of healthy dogs (p<0.05) and (p<0.01), respectively. The mean initial plasma D-dimer level was 2.84±0.50 and 2.99±0.61 ng/L in treated and untreated control groups. At the final follow-up evaluation on day 10 was 0.27±0.50 and 2.14±0.61 ng/L, in treated and untreated control groups, respectively, which was significantly lower in treated group (p<0.001). The area under curve (AUC) of the receiver operating characteristics for d-dimer was 0.922 (z-value =12.977, p<0.0001). (95%CI: 0.780–0.885). At a cut-off value of 0.1 ng/L, the D-dimer measurement had a sensitivity of 87.2%, a specificity of 90.9%. There was a correlation between D-dimer levels and logarythmic cyst counts.
Biochemical markers are now being closely scrutinized for detecting disease activity. D-dimer, as a valuable and non-invasive thrombotic predictor, has currently been investigated for supporting a connection between thrombosis and inflammation along with other parameters. As a result, these D-dimer concentrations measured in this disease were concluded to be a pre-biomarker in the diagnosis of these and similar diseases. It should not be unwise to draw preliminary conclusions that D-dimer levels support the link between probable thrombosis and inflammation, namely low grade.
Acknowledgements
The present study was not supported by any financial enterprenieur and self funded by researcher group.
REFERENCES
1. Bartelt LA, Sartor RB. Advances in understanding Giardia: Determinants and mechanisms of chronic sequelae. F1000Prime Rep. 2015; 7:62.
2. Buret A, Amat C, Manko A, Beatty J, Halliez MM, Bhargava A, et al. Giardia duodenalis: New research developments in pathophysiology, pathogenesis, and virulence factors. Curr Trop Med Rep. 2015; 2(3):110–118.
3. Maizels RM. Parasite immunomodulation and polymorphisms of the immune system. J Biol. 2009; 8:62.
4. McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012; 25:585–608.
5. Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(−/−) mice. Am J Pathol. 2013; 182:852–865.
6. Hanevik K, Hausken T, Morken MH, Strand EA, Mørch K, Coll P, Helgeland L, Langeland N. Persisting symptoms and duodenal inflammation related to Giardia duodenalis infection. J Infect. 2007; 55(6):524-530.
7. Sara D, Christine L, Kim N, Raymond D, Elaine H, Lars E. Novel model of colitis induced by a non-inflammatory small bowel infection. J Immunol 2011; 186: (1 Supplement) 166.10.
8. Chen TL, Chen S, Wu HW, Lee TC, Lu YZ, Wu LL, et al.. Persistent gut barrier damage and commensal bacterial influx following eradication of Giardia infection in mice. Gut Pathog. 2013; 5:26.
9. Benere E, Van Assche T, Van Ginneken C, Peulen O, Cos P, Maes L. Intestinal growth and pathology of Giardia duodenalis assemblage subtype a(i), a(ii), b and e in the gerbil model. Parasitol. 2012; 139:424–433.
10. Dos Santos JI, Vituri Cde L. Some hematimetric findings in human Giardia lamblia infection. Rev Inst Med Trop. 1996; 38:91–95.
11. Koot BG, Kate FJ, Juffrie M, Rosalina I, Taminiau JJ, Benninga MA. Does Giardia lamblia cause villous atrophy in children?: A retrospective cohort study of the histological abnormalities in giardiasis. J Pediatr Gastroenterol Nutr. 2009; 49:304–308.
12. Aloisio F, Filippini G, Antenucci P, Lepri E, Pezzotti G, Caccio SM, Pozio E. Severe weight loss in lambs infected with Giardia duodenalis assemblage b. Parasitol. 2006; 142:154–158.
13. Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, Hoffman P, Warren C, Singer S, Guerrant R. Persistent Giardia lamblia impairs growth in a murine malnutrition model. J Clin Investig. 2013; 123:2672–2684.
14. Jimenez JC, Fontaine J, Grzych JM, Dei-Cas E, Capron M. Systemic and mucosal responsesto oral administration of excretory and secretory antigens from Giardia intestinalis. Clin Diagn Lab Immunol. 2004; 11:152-160.
15. Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, Turk D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012; 1824:68–88.
16. Zareie M, McKay DM, Kovarik GG, Perdue MH. Monocyte/macrophages evoke epithelial dysfunction: Indirect role of tumor necrosis factor-alpha. Am J Physiol. 1998; 275:932-939.
17. Davison AM, Thomson D, Robson JS. Intravascular coagulation complicating influenza A virus infection. Br Med J. 1973; 1:654-655.
18. Saibeni S, Cattaneo M, Vecchi M, Zighetti ML, Lecchi A, Lombardi R, et al. Low vitamin B6 plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenter. 2003; 98(1):112–117.
19. Saibeni S, Spina L, Vecchi M. Exploring the relationships between inflammatory response and coagulation cascade in inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2004; 8(5):205-208.
20. Pastorelli L. Salvo CD, Mercado J R, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013; 4:280.
21. Pastorelli L, Dozio E, Francesca LP, Anzoletti MB, Vianello E, Munizio N, et al. Procoagulatory state in inflammatory bowel diseases is promoted by impaired intestinal barrier function. Gastroenterol Res Pract. 2015; Article ID 189341.
22. Ankarklev J, Jerlström-Hultqvist J, Ringqvist E, Troell K, Svärd SG. Behind the smile: cell biology and disease mechanisms of Giardia species. Nat Rev Microbiol. 2010; 8:413-422.
23. Buret AG. Pathophysiology of enteric infections with Giardia duodenalis. Parasite. 2008; 15:261-265.
24. Buret AG, Mitchell K, Muench DG, Scott KG. Giardia lamblia disrupts tight junctional ZO-1 and increases permeability in non-transformed human small intestinal epithelial monolayers: effects of epidermal growth factor. Parasitol. 2002; 125:11-19.
25. Cotton JA, Beatty JK, Buret AG. Host parasite interactions and pathophysiology in Giardia infections. Int J Parasitol. 2011; 41:925-933.
26. Teoh DA, Kamieniecki D, Pang G, Buret AG. Giardia lamblia rearranges F-actin and alpha-actinin in human colonic and duodenal monolayers and reduces transepithelial electrical resistance. J Parasitol. 2000; 86:800-806.
27. Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, Jelinek T, Zeitz M, Fromm M, Schulzke JD. Effect of chronic Giardia lamblia infection on epithelial trans- port and barrier function in human duodenum. Gut. 2007; 56:328-335.
28. Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterol. 2002; 123:1179-1190.
29. Yuan SM, Shi YH, Wang JJ, Lü FQ, Gao S. Elevated plasma D-dimer and hypersensitive C-reactive protein levels may indicate aortic disorders. Rev Bras Cir Cardiovasc. 2011, 26(4):573-81.
30. Ural DA, Ayan A, Aysul A, Balıkçı C, Ural K. Secnidazol treatment to improve milk yield in sheep with giardiasis. Atatürk Üniversitesi Vet Bil Derg. 2014, 9(2):74-82.